The most common techniques includes the basic wet chemistry techniques, atomic absorption spectroscopy (AAS), Inductive Coupled Plasma (ICP) techniques like ICP-OES & ICP-MS, as well as X-Ray Fluorescence Spectroscopy (XRF).
X-Ray fluorescence is a fast and non-destructive analysis technique, which usually allows for minimal sample preparation. Applications are very broad, spanning across various industries, such as the Metals and Mining industry, Cements, Foods, Oil and Gas, Polymers and Plastics, Minerology and geology, to niche applications, such as thickness analysis in the semiconductor industry.
Unlike the competing elemental analysis techniques, x-ray fluorescence technique is highly versatile in terms of sample types, the technique does not impose a requirement to digest the sample into liquid form. This removes one of the key challenges for the analysis of refractory materials, for example, Zircon. Zircon belongs to the group of neosilicates, with the chemical name of zirconium (IV) Silicate, ZrSiO4. The chemical is notorious for chemical inertness, being resistant to the common mineral acids, thus difficult to digest and analyze as a liquid sample. Adding to this, the zircon mineral is relatively hard, scaling at 7.5 on the Mohs scale, making it a difficult material to grind. It is only soluble using drastic chemical and physical process, with the most common method involving chemical treatment with Hydrofluoric acid (HF), due to its well-known ability to react with SiO2, which is also part of the zircon matrix. This method however carries the inherent safety risks of using hydrofluoric acid, as well as risks to the instrument parts containing glass.
The XRF spectroscopy avoids this route entirely, by analyzing the sample as a solid. Generally, powdered mineral samples could be analyzed as is in loose powder form, as a pressed pellet, or prepared as a fused bead. In this discussion, we will focus on the comparison between the pressed pellet and the fused bead sample preparation method.
Pressed pellet method involves compacting samples under high pressure, typically in the range of 15 tons to 40 tons. This step greatly improves reproducibility by minimizing packing density variation. However, pressed pellets are inherently a heterogeneous sample. XRF spectroscopy does not cover the entire volume of the sample (see Figure 1), thus the heterogeneity carries some negative properties, which cannot be eliminated. This includes particle size variations, varying minerology, surface roughness, and segregation, which will impact the detected fluorescence intensities. These factors can change as the raw material source changes, and as the upstream grinding equipment and sample preparation equipment age, which negatively impacts both the quality of calibration, as well as the confidence in the final results as the process condition changes.
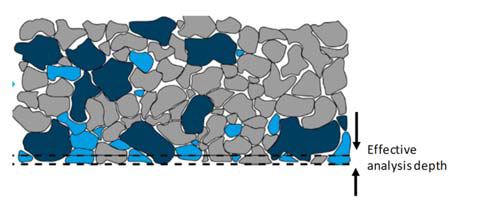
Figure 1: Depiction of a heterogeneous sample and the impact of limited analysis depth. Different colors represents the different possible mineral present
In the fused bead sample preparation method, a homogeneous sample is obtained by dissolving various oxides samples into borate salts at high temperature, typically around 1000°C. This step eliminates all of the negative properties of the pressed pellets. However, this method introduces dilution to the sample’s intensity, thus reducing the instrument’s sensitivity to low level trace detections.

Figure 2: Various different sample types. From left to right: unprepared samples, ground samples/loose powder, Pressed Pellets and Fused (Glass)
In this study, an Epsilon 3 EDXRF belonging to a customer was used, along with a single position LeNeo fusion instrument. The Epsilon 3 EDXRF comes with 30kV and 1mA excitation potential, at a maximum power of 9W with varying combinations. This allows the spectrometer to be tuned based on the specific elements of interest, optimizing it’s excitation potential and minimizing interfering elements. The LeNeo is a fully automatic cold to cold fusion instrument, with virtually unlimited programmable heating and cooling steps, allowing for a highly reproducible and reliable sample preparation step. A set of standards were provided by our customer, which was prepared and verified using an atomic absorption technique & Wet chemistry analysis. For the fused bead sample preparation, the samples were prepared in a 1:10 ratio of sample:flux, using the flux Lithium Tetraborate+Lithium Metaborate with the ratio of 50/50, and a small amount of premix Lithium Iodide. The pressed pellet samples were pressed with a 20 tons press to form a solid pellet.
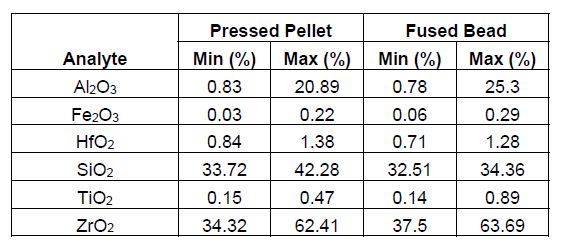
Table 1: Calibration range for various elements
Figure 3 and Figure 4 gives a comparison in terms of calibration quality between preparation as fused beads and pressed pellet. Figure 3 compares the calibration of major elements present in the samples, while Figure 4 compares the calibration of minor elements in zircon samples. The RMS or standard error quantifies the residual errors of the calibration line, where a lower value indicates a lower overall error for the calibration. For Zircon, the fused bead preparation provides for a lower overall RMS value and a better R2 value of 0.078987 and 0.999969 respectively, with about 4.7x lower error as compared to the pressed pellet calibration. The difference is larger for lighter elements, which is clearly demonstrated by the calibration of Aluminium. In this case, a difference in RMS of approximately 6x is observed, favouring the fused bead preparation.
Despite the higher dilution of fused beads as compared to pressed pellets, the former is still highly reliable for the analysis of minor elements. In this study, the analyte Iron Oxide, Fe2O3 and Titanium Dioxide, TiO2 is used as an example, with a calibration range of 0.06 wt.% to 0.29 wt.% and 0.14 wt. % to 0.89 wt. % respectively. In this study, the error is approximately 2x lower in the fused bead calibrations. A much better correlation factor is obtained for both analytes used in this example.
The results obtain in this document demonstrates the feasibility of quality and process control of refractory materials, without going through the process of digestion using hazardous chemicals. Additionally, the use of fused bead as a sample preparation methodology greatly improves the accuracy and reliability of XRF analysis.
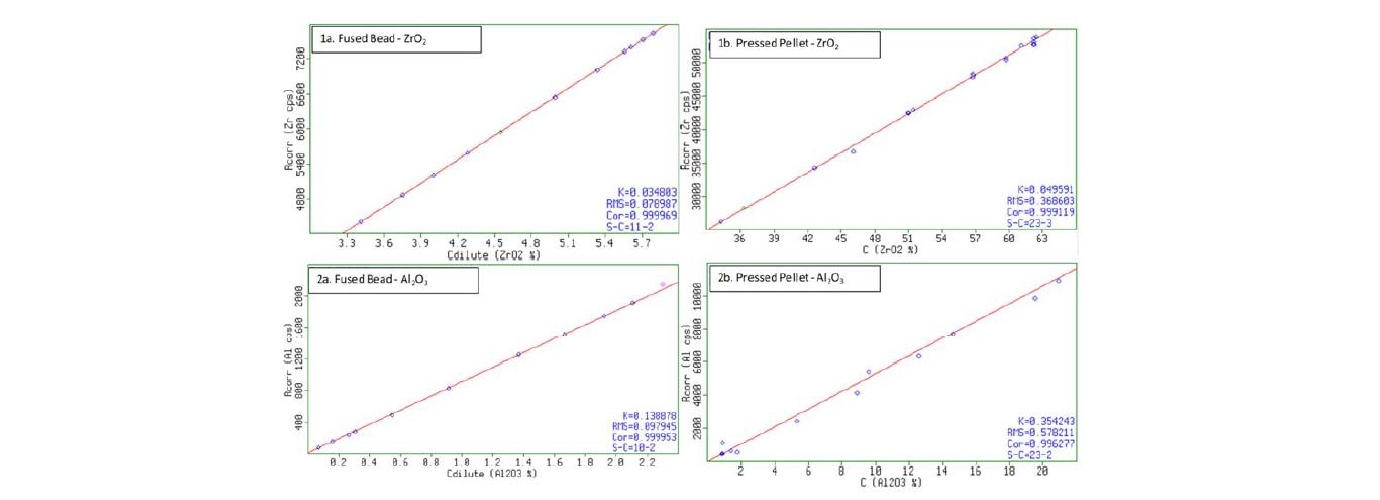
Figure 3: Comparison between Fused Beads and Pressed Pellet - Major Elements
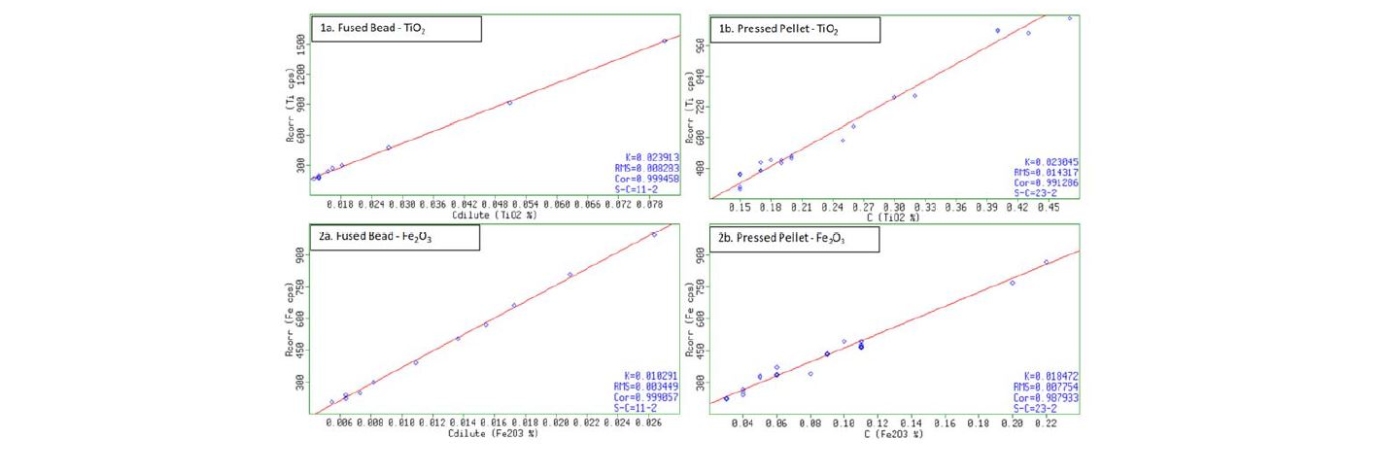
Figure 4: Comparison between Fused Beads and Pressed Pellet - Minor Elements